A water molecule consists of one oxygen atom covalently bonded to two hydrogen atoms.
- the electrons are not shared perfectly evenly: the oxygen atom is capable of pulling them towards itself and further away from the hydrogen atoms.
- The result is that the oxygen part of the molecule becomes slightly negatively charged, and the hydrogen atoms slightly positively charged. (this makes oxygen the more electronegative atom)

Water is therefore described as a polar molecule (polar means: one end of the molecule has a partial positive charge while the section has a partial negative charge / means: charged internally).
Two atoms, connected by a covalent bond, may exert different attractions for the electrons of the bond. In such cases the bond is polar, with one end slightly negatively charged and the other slightly positively charged.
DELTA (δ)
A partial charge. δ− represents a negative partial charge, and δ+ represents a positive partial charge chemistry
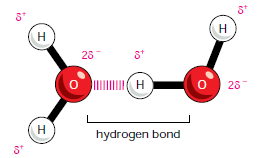
HYDROGEN BONDS
- Because they are polarized, two adjacent H2O molecules can form a linkage known as a hydrogen bond.
- A hydrogen bond is NOT A BOND because no electrons are involved, instead it is a FORCE OF ATTRACTION.
- Hydrogen bonds have only about 1/20 the strength of a covalent bond.
- Hydrogen bonds are strongest when the three atoms lie in a straight line.
WATER STRUCTURE
- Molecules of water join together transiently in a hydrogen-bonded lattice.
- Even at 37oC, 15% of the water molecules are joined to four others in a short-lived assembly known as a “flickering cluster.”
- The cohesive nature of water is responsible for many of its unusual properties, such as high surface tension, specific heat, and heat of vaporization.
Substances that dissolve readily in water are termed hydrophilic.
- They are composed of ions or polar molecules that attract water molecules through electrical charge effects.
- Water molecules surround each ion or polar molecule on the surface of a solid substance and carry it into solution.
HYDROPHOBIC MOLECULES
Molecules that contain a preponderance of non-polar bonds are usually insoluble in water and are
termed hydrophobic.
This is true, especially, of hydrocarbons, which contain many C–H bonds.
- Water molecules are not attracted to such molecules and so have little tendency to surround them and carry them into solution.
- This is known as formation of micelles. One very good example is shown below.








I think this is a wonderful story! You positively know the best way to convey an issue to mild and make it important. Extra individuals have to read this and perceiv…
ReplyDeletebiochemistry
This site is dedicated to helping you understand and treat issues regarding imbalanced methylation and therefore your state of health.