Proteins
Proteins are organic molecules consisting of many amino acids bonded together.Amino Acids: Monomers or building blocks of all proteins.
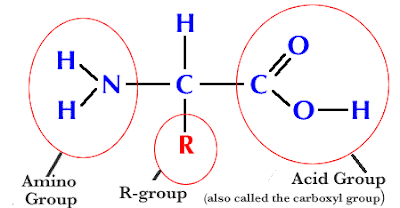
Parts of the Amino Acid:
a) Amino group (NH2)
b) Carboxyl group (COOH)
c) R-group: variable- 20 R-groups, so only 20 amino acids.
Primary structure - Proteins are made up of polypeptide chains, which are amino acids joined together with peptide bonds. The unique sequence of amino acids that make up a protein or polypeptide chain is called the Primary Structure.
- Peptide bonds are created by enzyme catalysed condensation reactions and broken down by enzyme catalysed hydrolysis reactions. Breaking down proteins is important in many areas of the body, not merely in digestion. For example, in hormone regulation, cells that are targeted by hormones contain enzymes to break down those hormones. This stops their effects from being permanent and allows them to be controlled.
Peptide bonds: Bond formed when 2 amino acids bond by condensation synthesis (See diagram below.)
Dipeptide- 2 amino acids joined by peptide bond.
Polypeptide- many amino acids bonded together.

- Secondary Structure - After synthesis, polypeptide chains are folded or pleated into different shapes, called their Secondary Structure. Two common examples of secondary structures are Alpha Helices and Beta Pleated Sheets. Secondary structure is held together by many Hydrogen bonds, overall giving the shape great stability.
- The final 3D structure of a protein is its Tertiary Structure, which pertains to the shaping of the secondary structure. This may involve coiling or pleating, often with straight chains of amino acids in between.
- Tertiary structure is held together by four different bonds and interactions:
- Disulphide Bonds - Where two Cysteine amino acids are found together, a strong double bond (S=S) is formed between the Sulphur atoms within the Cysteine monomers.
- Ionic Bonds - If two oppositely charged 'R' groups (+ve and -ve) are found close to each other, and ionic bond forms between them.
- Hydrogen Bonds - Your typical everyday Hydrogen bonds.
- Hydrophobic and Hydrophilic Interactions - Some amino acids may be hydrophobic while others are hydrophilic. In a water based environment, a globular protein will orientate itself such that it's hydrophobic parts are towards its centre and its hydrophilic parts are towards its edges
- Proteins with a 3D structure fall into two main types:
- Globular - These tend to form ball-like structures where hydrophobic parts are towards the centre and hydrophilic are towards the edges, which makes them water soluble. They usually have metabolic roles,for example: enzymes in all organisms, plasma proteins and antibodies in mammals.
- Fibrous - They proteins form long fibres and mostly consist of repeated sequences of amino acids which are insoluble in water. They usually have structural roles, such as: Collagen in bone and cartilage, Keratin in fingernails and hair.

Quaternary Structure: Some proteins are made up of multiple polypeptide chains, sometimes with an inorganic component (for example, a haem group in haemoglogin) called a Prosthetic Group. These proteins will only be able to function if all subunits are present.
- Haemoglobin is a water soluble globular protein which is composed of two α polypeptide chains, two β polypeptide chains and an inorganic prosthetic haem group. Its function is to carry oxygen around in the blood, and it is facilitated in doing so by the presence of the haem group which contains a Fe2+ ion, onto which the oxygen molecules can bind.
- Collagen is a fibrous protein consisting of three polypeptide chains wound around each other. Each of the three chains is a coil itself. Hydrogen bonds form between these coils, which are around 1000 amino acids in length, which gives the structure strength. This is important given collagen's role, as structural protein. This strength is increased by the fact that collagen molecules form further chains with other collagen molecules and form Covalent Cross Links with each other, which are staggered along the molecules to further increase stability. Collagen molecules wrapped around each other form Collagen Fibrils which themselves form Collagen Fibres.
- Collagen has many functions:
- Form the structure of bones
- Makes up cartilage and connective tissue
- Prevents blood that is being pumped at high pressure from bursting the walls of arteries
- Is the main component of tendons, which connect skeletal muscles to bones
- Haemoglobin may be compared with Collagen as such:
- Basic Shape - Haemoglobin is globular with four chains while Collagen is fibrous with three chains
- Solubility - Haemoglobin is soluble in water while Collagen is insoluble
- Amino Acid Constituents - Haemoglobin contains a wide range of amino acids while Collagen has 35% of it primary structure made up of Glycine
- Prosthetic Group - Haemoglobin contains a haem prosthetic group while Collagen doesn't possess a prosthetic group
- Tertiary Structure - Much of the Haemoglobin molecule is wound into α helices while much of the Collagen molecule is made up of left handed helix structures
Functions of Proteins & Named Examples
2) Defense: Antibodies - Globular proteins that "recognize" foreign microbes.
3) Transport- Hemoglobin (red blood cell protein).
4) Structure / Support- Collagen, which forms the matrix of skin, ligaments, tendons and bones.
5) Motion- Actin, a muscle protein responsible for muscle contraction.
6) Regulation- Hormones which serve as intercellular messengers. Example -Insulin (blood sugar regulation).
Denaturation: protein shape altered with changes in pH, temperature. Change in shape alters activity of enzyme. Enzymes function within a narrow range of these factors. The organised strucutre of protein is affecting by denaturation in varied ways:
1. In Primary Structure: the sequence of amino acids held together by covalent peptide bonds, is not disrupted by denaturation.
2. In Secondary Structure: denaturation, proteins lose all regular repeating patterns such as alpha-helices and beta-pleated sheets, and adopt a random coil configuration
3. In Tertiary structure: denaturation involves the disruption of:
• Covalent interactions between amino acid side-chains (such as disulfide bridges between cysteine groups)
• Noncovalent dipole-dipole interactions between polar amino acid side-chains (and the surrounding solvent)
• Van der Waals (induced dipole) interactions between nonpolar amino acid side-chains.
4. In quaternary structure: Denaturation, protein sub-units are dissociated and/or the spatial arrangement of protein subunits is disrupted.
Denaturing Agents
|
Denaturing
Agents
|
Explanation
of denaturation
|
|
Temperature
|
Heat can be used to disrupt hydrogen bonds and non-polar hydrophobic
interactions. This occurs because heat increases the kinetic energy and
causes the molecules to vibrate so rapidly and violently that the bonds are
disrupted. Eg. When eggs coagulate when fried or boiled
|
|
pH
|
When the pH is adjusted to the normal isoelectric point for protein,
its net charge will be zero (zwitterion). If the pH is lowered far below the
isoelectric point, the protein will lose its negative charge and contain only
positive charges. The like charges will repel each other and prevent the
protein from aggregating as readily, preventing formation of amine bonds
|
|
Acids and bases
|
acids and bases disrupt salt bridges held together by ionic charges
|
|
Alcohols
|
Hydrogen bonding occurs between amide groups in the secondary protein
structure. Hydrogen bonding between "side chains" occurs in
tertiary protein structure in a variety of amino acid combinations. All of
these are disrupted by the addition of another alcohol.
|
|
Heavy metal salts
|
Heavy metal salts act to denature proteins in much the same manner as
acids and bases. Heavy metal salts usually contain Hg+2, Pb+2, Ag+1 Tl+1,
Cd+2 and other metals with high atomic weights. Since salts are ionic they
disrupt salt bridges in proteins. The reaction of a heavy metal salt with a
protein usually leads to an insoluble metal protein salt. Eg. Disulphide bridges
are easily disrupted this way
|
|
Reducing agents
|
If oxidizing agents cause the formation of a disulphide bond, then
reducing agents, of course, act on any disulphide bonds to split it apart.
|
|
Detergents
|
·
hydrophobic parts of the detergent associate
with the hydrophobic parts of the protein (coating with detergent molecules)
·
hydrophilic ends of the detergent molecules
interact favourably with water (nonpolar parts of the protein become coated
with polar groups that allow their association with water)
·
hydrophobic parts of the protein no longer
need to associate with each other
·
Dissociation of the non-polar R groups can
lead to unfolding of the protein chain (same effect as in nonpolar solvents).
|
|
Agitation
|
Whipping action stretches the polypeptide chain until the bonds break.
|




No comments:
Post a Comment